György Tegze1, Tamás Pusztai1, Gyula Tóth2, László Gránásy1,3, A Svandal, T Buanes, T Kuznetsova, Bjørn Kvamme4
1Institute for Solid State Physics and Optics, Wigner Research Centre for Physics, P.O. Box 49, Budapest H-1525, Hungary
2Department of Mathematical Sciences, Loughborough University, Loughborough, Leicestershire, LE11 3TU, U.K.
3BCAST, Brunel University, Uxbridge, Middlesex, UB8 3PH, United Kingdom
4Institute of Physics and Technology, University of Bergen, Allégaten 55, N-5007 Bergen, Norway
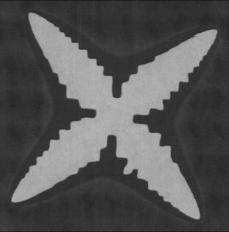
A phase field theory with model parameters evaluated from atomistic simulations/experiments is applied to predict the nucleation and growth rates of solid CO2 hydrate in aqueous solutions under conditions typical to underwater natural gas hydrate reservoirs. It is shown that under practical conditions a homogeneous nucleation of the hydrate phase can be ruled out. The growth rate of CO2 hydrate dendrites has been determined from phase field simulations as a function of composition while using a physical interface thickness 0.85±0.07 nm evaluated from molecular dynamics simulations. The growth rate extrapolated to realistic supersaturations is about three orders of magnitude larger than the respective experimental observation. A possible origin of the discrepancy is discussed. It is suggested that a kinetic barrier reflecting the difficulties in building the complex crystal structure is the most probable source of the deviations.


